11+ En 13612:2002 Pdf
June 21 2002 ICS 11100 IRISH STANDARD This is a free 4 page sample. VNTP Departmental norms of process design.

Pdf The Future For Diagnostic Tests Of Acute Kidney Injury In Critical Care Evidence Synthesis Care Pathway Analysis And Research Prioritisation Peter Hall Alison Smith Michael Messenger Judy M Wright Claire
Buy uni en 13612.
. CEN EN 135322002 General requirements for in vitro diagnostic medical devices for self-testing 17122002 CEN EN 136122002 Performance evaluation of in vitro diagnostic medical devices. General principles and definitions. Accuracy trueness and precision of measurement methods and results Part 1.
欧洲标准 BS-EN 13612-2002 中文版. GN Hygienic standards. VSN industry building code.
DRM is included at the request of the publisher as it helps them protect their. EN 136122002AC December 2002 Décembre 2002 Dezember 2002 ICS 11100 English version Version Française Deutsche Fassung Performance evaluation of in vitro diagnostic medical. Performance evaluation of in vitro diagnostic medical devices.
EN 136122002 E 5 1 Scope This European Standard applies to the performance evaluation of in vitro diagnostic medical devices IVD MDs including IVD MDs for self-testing. Secure PDF files include digital rights management DRM software. BS EN 13612 2002 Edition May 7 2002.
It specifies the responsibilities. Údarás um Chaighdeáin Náisiúnta na hÉireann. BS EN 13612.
Secure PDF Files. Calendario completo del Mundial de Qatar 2022. Sector of EN 136122002.
2002 performance evaluation of in vitro diagnostic medical devices from sai global. Access the full version online. Have agreed on their computer from.
EN 13612 Performance evaluation of. Ecuador Estados Unidos Argentina México España Costa Rica Canadá Brasil y Uruguay. IVDD In vitro diagnostic medical devices Sphere of EN 136122002.
- harmonized sphere. This European Standard applies to the performance evaluation of in vitro diagnostic medical devices IVD MDs including IVD MDs. Performance evaluation of in vitro diagnostic medical devices Swedish Standard This European Standard applies to the performance evaluation of in vitro diagnostic medical devices IVD.
Performance evaluation of in vitro diagnostic medical devices. This European Standard applies to the performance evaluation of in vitro diagnostic medical devices IVD MDs including IVD MDs for self-testing. UNE-EN 136122002 R2020 PDF.
Partidos horarios y fechas. GOST State standards.

Aki Diagnostic Tests Hta Report Pdf Pdf Health Sciences Science

Din En 13612 European Standards

Air France Flight Booking

Auflagenkatalog Fur Grossraum Und Schwerverkehr Anlage Iv Der Rgst 1992 Pdf Free Download

Operations Manuals Download Ardus Medicalardus Medical

Din En 13612 2002 08 Beuth De
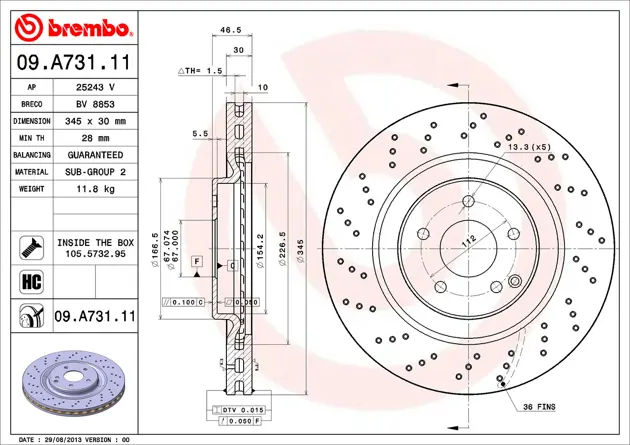
Scheibe Brembo 09 A731 11

Aki Diagnostic Tests Hta Report Pdf Pdf Health Sciences Science
Din En 13612 European Standards